Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 , He
, He
 , C
, C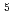
 and Ne
and Ne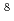
 ions
ionsIwamatsu, Kazuhiro; Yamashita, Shinichi*; Taguchi, Mitsumasa; Kimura, Atsushi; Kurashima, Satoshi; Katsumura, Yosuke
no journal, ,
Heavy ion beams, one of the high linear energy transfer (LET) radiations induce specific irradiation effects which are different from those of low LET radiations. The effects are attributed to heterogeneous distribution of reactive species along their trajectories, so called "track structure". Water was selected as target in this study because more data exist for radiolysis than any other substances. Hydroxyl radical (OH), one of the most important water decomposition species, was focused on by using bromide ion as a probing reagent, and their reactions were observed by the ion beam pulse radiolysis system. The formation and decay of Br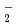 were observed at 375 nm (
were observed at 375 nm ( [Br
[Br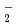 ] = 9000 M
] = 9000 M
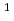 cm
cm
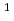 ). The formation chemical yield of it after pulse irradiation decreased with increasing atomic numbers of the incident ions. Radical recombination reaction occurs easier by higher LET ions because of denser radical formations. Therefore, the decrease in the yields of OH scavenged by Br
). The formation chemical yield of it after pulse irradiation decreased with increasing atomic numbers of the incident ions. Radical recombination reaction occurs easier by higher LET ions because of denser radical formations. Therefore, the decrease in the yields of OH scavenged by Br induces the decrease in the chemical yields of Br
induces the decrease in the chemical yields of Br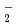 . The decay rates of the chemical yields increased with the atomic mass of the ions. The concentrations depend on the dose rate and chemical yield, and the dose rates proportional to LET value. The concentrations after pulse increased with increase in the atomic mass of the ions and resulted in faster decay in heavier ions.
. The decay rates of the chemical yields increased with the atomic mass of the ions. The concentrations depend on the dose rate and chemical yield, and the dose rates proportional to LET value. The concentrations after pulse increased with increase in the atomic mass of the ions and resulted in faster decay in heavier ions.